藥品名稱
drug name | IMOVAX POLIO 0.5mL/syringe (Poliovirus vaccine (inactivated)), 巴斯德小兒麻痺注射疫苗 |
| 藥檔狀態 | 使用中 |
成 份
Ingredient | Poliovirus vaccine (inactivated) |
| 單位含量 | 0.5mL/syringe |
| Dosage Forms | Injection, suspension: Type 1 poliovirus 40 D-antigen units, type 2 poliovirus 8 D-antigen units, and type 3 poliovirus 32 D-antigen units per 0.5mL |
| 外觀描述 | |
| Appearance | |
廠商名稱
Manufacturer | 賽諾菲股份有限公司 |
製 造 商
Manufacturer | SANOFI PASTEUR |
字 號
Product ID | 衛署菌疫輸字第000440號 |
藥理分類
Pharmacologic Category | Vaccine; Vaccine, Inactivated (Viral) |
作用機轉
Mechanism of action | As an inactivated virus vaccine, poliovirus vaccine induces active immunity against poliovirus types 1, 2, and 3 infection. |
| 用途/適應症 | |
| Use |
Poliovirus prevention:
- Active immunization of infants (≧6 weeks [US labeling]; ≧2 months [Canadian labeling]), children, adolescents, and adults for prevention of poliomyelitis caused by poliovirus types 1, 2, and 3.
‧ US labeling: Infants (as young as 6 weeks), children, adolescents, and adults
‧ Canadian labeling: Infants (as young as 2 months), children, adolescents, and adults
- The Advisory Committee on Immunization Practices (ACIP) recommends routine vaccination for the following:
‧ All infants and children (first dose given at 2 months of age) (CDC/ACIP, 58[30] 2009)
Routine immunization of adults in the United States is generally not recommended. Adults with previous wild poliovirus disease, who have never been immunized, or those who are incompletely immunized may receive inactivated poliovirus vaccine if they fall into one of the following categories (CDC/ACIP [Prevots 2000]):
‧ Travelers to regions or countries where poliomyelitis is endemic or epidemic
‧ Healthcare workers in close contact with patients who may be excreting poliovirus
‧ Laboratory workers handling specimens that may contain poliovirus
‧ Members of communities or specific population groups with diseases caused by wild poliovirus
‧ Incompletely vaccinated or unvaccinated adults in a household or with other close contact with children receiving oral poliovirus (may be at increased risk of vaccine associated paralytic poliomyelitis)
|
衛福部核准適用症狀
MOHW approved indications |
預防小兒麻痺症。
|
| 常用劑量 |
(藥品劑量會因人或病情增減,請依照醫師指示服用。) |
| Dose |
【Adult】
Immunization: IM, SubQ:
- Previously unvaccinated: Administer 0.5 mL per dose for a total of 3 doses given as follows: Two 0.5 mL doses administered at 1- to 2-month intervals, followed by a third dose 6 to 12 months later. If <3 months, but at least 2 months are available before protection is needed, 3 doses may be administered at least 1 month apart. If administration must be completed within 1 to 2 months, give 2 doses at least 1 month apart. If <1 month is available, give 1 dose.
- Incompletely vaccinated: Adults with at least 1 previous dose of OPV, <3 doses of IPV, or a combination of OPV and IPV equaling <3 doses, administer at least one 0.5 mL dose of IPV. Additional doses to complete the series may be given if time permits.
- Completely vaccinated and at increased risk of exposure: One 0.5 mL dose.
【Pediatric】
Primary immunization:Note: Use of the minimum age and minimum intervals (4 weeks) during the first 6 months of life should only be done when the vaccine recipient is at risk for imminent exposure to circulating poliovirus (shorter intervals and earlier start dates may lead to lower seroconversion) (CDC/ACIP 2009). Refer to CDC/ACIP for guidance on schedules and interval for individuals who received polio vaccination(s) (including oral formulation) outside of the US (CDC/ACIP [Marin 2017]).
- Infants and Children 6 weeks to 47 months: IM, SubQ: 0.5 mL per dose for a total of 3 doses administered as follows: 2 months, 4 months, and 6 to 18 months of age.
Booster immunization: Children 4 to 6 years: IM, SubQ: 0.5 mL as a single dose; administered at least 6 months from the previous dose.
Catch-up immunization: Infants, Children, and Adolescents 4 months to 18 years: Note: Do not restart the series if doses have been given (OPV and/or IPV) refer to current immunization guidelines for specific schedule and timing of dose based on patient age and previous number of doses. IM, SubQ: 0.5 mL per dose for a total of 1 to 4 doses.
【Geriatric】
Refer to adult dosing.
【Renal Impairment】
There are no dosage adjustments provided in the manufacturer`s labeling.
【Hepatic Impairment】
There are no dosage adjustments provided in the manufacturer`s labeling.
|
懷孕分級
Pregnancy Risk Factor |
依文獻內容判定系統稽核懷孕建置為:C
UpToDate:
Animal reproduction studies have not been conducted. Although adverse effects of IPV have not been documented in pregnant women or their fetuses, vaccination of pregnant women should be avoided on theoretical grounds. Pregnant women at increased risk for infection and requiring immediate protection against polio may be administered the vaccine (CDC/ACIP [Prevots 2000]).
仿單:
在高風險的情況下,於懷孕期間可施打本疫苗。
-----------------------------------------------------------
[FDA(美國食品及藥物管理局)懷孕分級說明:
A:對照試驗無法證實懷孕初期及後期使用會危害胎兒。
B:動物試驗無法證實對胎兒有害,但缺乏人類對照試驗;或動物試驗有副作用報告,但無法證實對懷孕初期及後期之胎兒有害。
C:動物實驗中對胎兒有害但缺乏孕婦對照實驗;或無動物及孕婦試驗。
D:證實對胎兒有害,但疾病對孕婦有生命威脅或較安全藥品無法使用或無效時可使用。
X:證實對胎兒有害,且使用後危害大於可能的益處。孕婦及可能懷孕婦女禁用。]
|
| 禁忌症 |
如有以下情況時,請勿使用IMOVAX POLIO疫苗:
1.對疫苗中之主成分或其他成份、neomycin、streptomycin和polymyxine B過敏。
2.先前施打本疫苗或含相同成分的疫苗後有過敏反應。
3.有發燒或急性疾病時,應延後使用疫苗。
|
| Contraindications |
Hypersensitivity to any component of the vaccine, including 2-phenoxyethanol, formaldehyde, neomycin, streptomycin and polymyxin B; anaphylaxis or anaphylactic shock occurring within 24 hours of administration of 1 dose of vaccine; acute, febrile illness (excluding minor illness with or without low-grade fever).
|
| 常見副作用 | |
| Common adverse drug reactions | |
| Adverse Reactions |
Percentages noted with concomitant administration of DTP or DTaP vaccine and observed within 48 hours of injection.
>10%:
- Central nervous system: Irritability (7% to 65%; most common in infants 2 months of age), fatigue (4% to 61%)
- Gastrointestinal: Anorexia (1% to 17%)
- Local: Tenderness at injection site (≦29%), swelling at injection site (≦11%)
1% to 10%:
- Central nervous system: Excessive crying (≦1%; reported within 72 hours)
- Gastrointestinal: Vomiting (1% to 3%)
- Local: Erythema at injection site (≦3%)
- Miscellaneous: Fever (>39°C: ≦4%)
<1%, postmarketing, and/or case reports: Agitation, anaphylactic shock, anaphylaxis, arthralgia, drowsiness, febrile seizures, headache, hypersensitivity reaction, lymphadenopathy, myalgia, paresthesia, seizure, skin rash, urticaria
|
★高警訊藥品
監測建議 |
|
監測
Monitoring |
Monitor for anaphylaxis and syncope for 15 minutes following administration (ACIP [Ezeanolue 2020]). If seizure-like activity associated with syncope occurs, maintain patient in supine or Trendelenburg position to reestablish adequate cerebral perfusion.
|
| 警語與注意事項 | |
| Warnings & precautions | |
| 針劑溶解條件 |
|
| 針劑稀釋條件 |
|
| 針劑不相容性 |
|
| 針劑施打條件 |
建議投與途徑為肌肉注射,或亦可皮下注射。(1100525仿單資料)
|
| 針劑保存安定性 |
應冷藏(2~8℃)且避光儲存。請勿冷凍。(1100525仿單資料)
|
最近修改日期時間
Updated | 5/31/2021 10:04:24 AM |
|
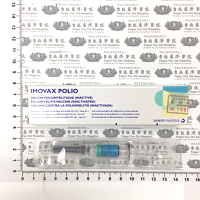
現用藥品
Available
|
停用藥品
Old item
|

藥品仿單
DrugLabeling
|
|

二維條碼
QR code
|
|