藥品名稱
drug name | Meningococcal Vaccine, Group A/C/Y/W-135, 0.5mL/vial (Nimenrix ,4價流行性腦脊髓膜炎結合型疫苗) |
| 藥檔狀態 | 停用 |
成 份
Ingredient | Meningococcal Polysaccharide Diphtheria Toxoid Conjugate Vaccine, Group A/C/Y/W-135 |
| 單位含量 | 0.5 mL/vial |
| Dosage Forms | Injection, solution [preservative free]:0.5mL/vial |
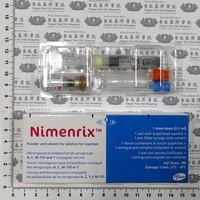
| 外觀描述 | |
| Appearance | |
廠商名稱
Manufacturer | Pfizer |
製 造 商
Manufacturer | GlaxoSmithKline Biologicals s.a. |
字 號
Product ID | 專案進口,無衛署藥字號 |
藥理分類
Pharmacologic Category | Vaccine;Vaccine, Inactivated (Bacterial) |
作用機轉
Mechanism of action | Induces immunity against meningococcal disease via the formation of bactericidal antibodies directed toward the polysaccharide capsular components of Neisseria meningitidis serogroups A, C, Y and W-135. |
| 用途/適應症 | |
| Use |
Labeled Indications:
Meningococcal disease prevention: Provides active immunization against invasive meningococcal disease caused by Neisseria meningitidis serogroups A, C, Y, and W-135 in individuals 6 weeks to 55 years of age.
The National Advisory Committee on Immunization (NACI) recommends:
- Routine vaccination with a monovalent or quadrivalent conjugate meningococcal vaccine in healthy children ≧12 years, adolescents, and young adults ≦24 years of age.
- Vaccination of high risk individuals:
>>> Adolescents and young adults
>>> Laboratory workers routinely exposed to isolates of N. meningitidis
>>> Military recruits
>>> Persons traveling to areas with high rates of endemic meningococcal disease or transmission. Use of a monovalent vaccine may not be appropriate based upon the serogroup prevalence in the region of travel.
>>> Persons with congenital complement, properdin, factor D or primary antibody deficiencies or with acquired complement deficiency due to receipt of the terminal complement inhibitor eculizumab
>>> Persons with HIV particularly if congenitally acquired
>>> Persons with anatomic or functional asplenia, including sickle cell disease
>>> Based on limited evidence and expert opinion, NACI considers vaccination with quadrivalent conjugate meningococcal vaccine appropriate in individuals ≧56 years of age who are at high risk of meningococcal disease due to travel to high risk areas or underlying medical conditions (NACI 2012).
- Postexposure management: NACI recommendations for persons considered to be at an increased risk for meningococcal disease:
>>> Chemoprophylaxis and immunoprophylaxis: Selection of meningococcal vaccination to be based upon serogroup(s):
‧ Individuals living in the same household, share sleeping arrangements, or with close contact (eg, kissing, shared cigarettes, shared eating or drinking utensils) of infected patient
‧ Employees and children of nursery schools or day care
Off-Label:
Postexposure management (close contacts) or outbreak control of meningococcal disease
|
衛福部核准適用症狀
MOHW approved indications |
專案進口,無衛署適應症。由疾管署專案進口用於國際旅遊醫療,預防流行性腦脊髓膜炎。
|
| 常用劑量 | 0
(藥品劑量會因人或病情增減,請依照醫師指示服用。) |
| Dose |
Adult
Meningococcal disease prevention:
- Manufacturer’s labeling: Adults ≦55 years: IM: 0.5 mL as single dose. Note: The need for a booster dose has not been established. Manufacturer labeling suggests that a second dose may be considered for patients at increased risk of exposure to meningococcal serogroup A and who received the first Nimenrix dose >1 year prior.
- Alternative recommendations (NACI 2012): Note: Based on limited evidence and expert opinion, use in high risk individuals ≧56 years of age is considered appropriate:
>>> Healthy individuals (≦24 years of age): IM: 0.5 mL as a single dose even if the individual has previously been vaccinated as an infant or toddler; dose should be limited to those not revaccinated as a child ≧12 years or adolescent.
>>> High-risk with underlying medical conditions and not previously immunized with quadrivalent conjugate meningococcal vaccine: IM: 0.5 mL dose; administer a total of 2 doses at least 8 weeks apart or at least 4 weeks apart when more rapid immunization is needed.
>>> Travel to high risk areas and not previously immunized with quadrivalent conjugate meningococcal vaccine: IM: 0.5 mL dose
Postexposure management (close contacts) or outbreak control (off-label use) (NACI 2012): Recommendations dependent on meningococcal serogroup involved:
- Serogroup C:
>>> Previously unvaccinated: IM: 0.5 mL/dose immediately after exposure (monovalent or quadrivalent vaccine may be used)
>>> Previously vaccinated: IM: If vaccinated at <1 year of age or if at high risk for invasive meningococcal disease due to underlying medical condition, re-vaccinate with 0.5 mL/dose if ≧4 weeks since last dose; otherwise re-vaccinate if at least 1 year since last dose (monovalent or quadrivalent vaccine may be used)
- Serogroup A, Y, or W-135:
>>> Previously unvaccinated: IM: 0.5 mL/dose immediately after exposure; high-risk individuals with underlying medical conditions routinely require a total of 2 doses (administered at least 8 weeks apart or at least 4 weeks apart when more rapid immunization is needed)
>>> Previously vaccinated: IM:
‧ If previously vaccinated with only monovalent conjugate meningococcal group C vaccine (Men C-C), administer 0.5 mL/dose (quadrivalent vaccine) immediately after exposure, regardless of when Men C-C was given
‧ If previously vaccinated with quadrivalent vaccine at <1 year of age or if at high risk for invasive meningococcal disease due to underlying medical condition, re-vaccinate with 0.5 mL/dose (quadrivalent vaccine) if ≧4 weeks since last dose; otherwise re-vaccinate if at least 1 year since last dose
衛生福利部疾病管制署 函 (發文日期:108.09.11)
說明:依據供應商 (Pfizer) 2019年2月更新仿單資訊:
追加接種建議:暴露於高風險環境者:建議追加接種1劑,且應與最後一次接種間隔至少5年。
Pediatric
Meningococcal disease prevention:
- Manufacturer’s labeling:
>>> Primary immunization:
‧ Infants 6 to 12 weeks of age: IM: 0.5 mL at 2 and 4 months of age (may start as early as 6 weeks of age)
‧ Children ≧1 year of age and Adolescents: IM: Refer to adult dosing.
>>> Booster immunization: IM: For infants who have completed the primary immunization 2-dose series, administer 0.5 mL as a single dose at 12 months of age. Note: The need for a booster dose in individuals primed with a single dose at ≧12 months of age has not been established. Manufacturer labeling suggests that a booster dose may be considered for patients at increased risk of exposure to meningococcal serogroup A and who received the first Nimenrix dose >1 year prior.
- Alternative recommendations (NACI 2012):
>>> Children ≧12 years of age and Adolescents: Healthy individuals: IM: 0.5 mL as a single dose administered routinely at 12 years of age, even if the individual has previously been vaccinated as an infant or toddler.
>>> Children ≧2 years of age and Adolescents:
‧ High-risk with underlying medical conditions and not previously immunized with quadrivalent conjugate meningococcal vaccine: Refer to adult dosing.
‧ Travel to high risk areas and not previously immunized with quadrivalent conjugate meningococcal vaccine: Refer to adult dosing.
Postexposure management (close contacts) or outbreak control (off-label use) (NACI 2012): Recommendations dependent on meningococcal serogroup involved:
- Serogroup C: Children ≧11 years of age and Adolescents: Refer to adult dosing.
- Serogroup A, Y, or W-135: Children ≧2 years of age and Adolescents: Refer to adult dosing.
衛生福利部疾病管制署 函 (發文日期:108.09.11)
說明:依據供應商 (Pfizer) 2019年2月更新仿單資訊:
(一) 接種年齡及劑次:
1. 6週已上且未滿6個月:接種2劑,且2劑應間隔至少2個月。
2. 6個月已上:接種1劑。
(二)追加接種建議:
1. 6週以上且未滿12個月之嬰幼兒:建議於滿12個月時追加接種1劑,且應與最後一次接種間隔至少2個月。
Renal impairment:
There are no dosage adjustments provided in the manufacturer`s labeling.
Hepatic impairment:
There are no dosage adjustments provided in the manufacturer`s labeling.
|
懷孕分級
Pregnancy Risk Factor |
依文獻內容判定系統稽核懷孕分級建置為:B
UpToDate:
Adverse effects were not observed in animal reproduction studies. According to the NACI, the use of conjugated meningococcal vaccines should be considered in pregnant women in circumstances such as travel to high risk areas or if indicated as postexposure prophylaxis against a vaccine preventable strain or during an outbreak (NACI 2012).
-----------------------------------------------------------
[FDA(美國食品及藥物管理局)懷孕分級說明:
A:對照試驗無法證實懷孕初期及後期使用會危害胎兒。
B:動物試驗無法證實對胎兒有害,但缺乏人類對照試驗;或動物試驗有副作用報告,但無法證實對懷孕初期及後期之胎兒有害。
C:動物實驗中對胎兒有害但缺乏孕婦對照實驗;或無動物及孕婦試驗。
D:證實對胎兒有害,但疾病對孕婦有生命威脅或較安全藥品無法使用或無效時可使用。
X:證實對胎兒有害,且使用後危害大於可能的益處。孕婦及可能懷孕婦女禁用。]
|
| 禁忌症 |
|
| Contraindications |
Hypersensitivity to any component of the vaccine.
|
| 常見副作用 | |
| Common adverse drug reactions | |
| Adverse Reactions |
>10%:
- Central nervous system: Irritability (infants: 52% to 63%; children: 15% to 41%), drowsiness (infants: 36% to 53%; children: 14% to 28%), fatigue (12% to 29%), headache (16% to 26%)
- Gastrointestinal: Anorexia (infants: 33% to 38%; children: 11% to 23%), gastrointestinal symptoms (≦15%; includes diarrhea, nausea, vomiting)
- Local: Pain at injection site (19% to 51%), erythema at injection site (infants & children: 25% to 43%; adolescents & adults: 9% to 26%), swelling at injection site (infants & children: 12% to 30%; adolescents & adults: 8% to 19%)
- Miscellaneous: Fever (≧38°C: 4% to 9%, infants: 23% to 32%; >40°C: <1%)
1% to 10%:
Local: Hematoma at injection site
<1%, postmarketing, and/or case reports: Crying, dizziness, erythema, hypoesthesia, induration at injection site, injection site reaction, insomnia, injection site pruritus, limb pain, local anesthesia (injection site), malaise, myalgia, pruritus, skin rash, swelling of injected limb, warm sensation at injection site
|
★高警訊藥品
監測建議 |
|
監測
Monitoring |
Monitor for syncope for at least 15 minutes following administration (ACIP [Kroger 2017]; NACI 2013). If seizure-like activity associated with syncope occurs, maintain patient in supine or Trendelenburg position to reestablish adequate cerebral perfusion.
|
| 警語與注意事項 | |
| Warnings & precautions | |
| 針劑溶解條件 |
需以所附的溶劑進行重組,將預充填好的溶劑的針筒接上針頭,刺入裝有乾粉的小瓶中,加入全部的溶劑後,搖晃小瓶使混合均勻。(1070831仿單資料)
|
| 針劑稀釋條件 |
|
| 針劑不相容性 |
|
| 針劑施打條件 |
肌肉注射。不可靜脈注射、皮下注射或皮內注射。(1070831仿單資料)
|
| 針劑保存安定性 |
避光儲存於2-8℃。不可冷凍。
配置好之疫苗若未立即施打,可於30℃以下存放8小時。(1070831仿單資料)
|
最近修改日期時間
Updated | 4/1/2021 8:53:02 AM |
|

現用藥品
Available
|
停用藥品
Old item
|

藥品仿單
DrugLabeling
|
|

二維條碼
QR code
|
|