藥品名稱
drug name | (公費) PENTAXIM 0.5mL/syringe, 潘多星五合一疫苗 |
| 藥檔狀態 | 使用中 |
成 份
Ingredient | 白喉、破傷風、百日咳、小兒麻痺以及b型嗜血桿菌 |
| 單位含量 | 0.5mL/syringe |
| Dosage Forms | Injection, suspension [preservative free]: 0.5mL |
| 外觀描述 | |
| Appearance | |
廠商名稱
Manufacturer | 賽諾菲股份有限公司 |
製 造 商
Manufacturer | SANOFI PASTEUR |
字 號
Product ID | 衛部菌疫輸字第000976號 |
藥理分類
Pharmacologic Category | Vaccine; Vaccine, Inactivated (Bacterial); Vaccine, Inactivated (Viral) |
作用機轉
Mechanism of action | Promotes active immunity to diphtheria, tetanus, pertussis, hepatitis B and poliovirus (types 1, 2 and 3) by inducing production of specific antibodies and antitoxins. |
| 用途/適應症 | |
| Use |
Diphtheria, tetanus, pertussis, poliomyelitis, and hepatitis B prevention: Active immunization against diphtheria, tetanus, pertussis, hepatitis B virus (all known subtypes), and poliomyelitis in infants born of HBsAg-negative mothers, beginning as early as 6 weeks of age through 6 years of age (prior to the seventh birthday).
The Advisory Committee on Immunization Practices (ACIP) recommends Pediarix for the following (CDC/ACIP [Liang 2018]; CDC/ACIP [Schillie 2018]; CDC/ACIP 58[30] 2009):
- Primary vaccination for DTaP, Hep B, and IPV in infants at 2, 4, and 6 months of age.
- To complete the primary vaccination series in children who have received DTaP (Infanrix) and who are scheduled to receive the other components of the vaccine. Whenever feasible, the same manufacturer should be used to provide the pertussis component; however, vaccination should not be deferred if a specific brand is not known or is not available. HepB and IPV from different manufacturers are interchangeable.
|
衛福部核准適用症狀
MOHW approved indications |
適用於出生2個月至7歲孩童的主動免疫接種,以預防白喉、破傷風、百日咳、小兒麻痺及侵襲性b型嗜血桿菌疾病。
對於其他類型之嗜血桿菌所引發的感染或其它微生物所造成的腦膜炎,本疫苗不具保護作用。
|
| 常用劑量 |
(藥品劑量會因人或病情增減,請依照醫師指示服用。) |
| Dose |
【Pediatric】
Note: Immunization during coronavirus disease 2019 (COVID-19) pandemic: Routine vaccination should not be delayed because of the COVID-19 pandemic (CDC 2020; WHO 2020). In general, simultaneous administration of all vaccines for which a patient is eligible (according to current immunization schedules/guidelines) is recommended (ACIP [Ezeanolue 2020]). However, vaccination of patients with suspected or confirmed COVID-19 infection (regardless of symptoms) should be postponed to avoid exposure to health care personnel and other patients (CDC 2020). Additional information is available from the CDC, the American Academy of Pediatrics, and the Immunization Action Coalition.
Note: Consult CDC/ACIP annual immunization schedules for additional information including specific detailed recommendations for catch-up scenarios and/or care of patients with high-risk conditions. According to ACIP, doses administered ≦4 days before minimum interval or age are considered valid; however, local or state mandates may supersede this timeframe (ACIP [Ezeanolue 2020]).
Primary immunization: Infants ≧6 weeks and Children <7 years: IM: 0.5 mL per dose for a total of three doses administered as follows: 2, 4, and 6 months of age in 6- to 8-week intervals (preferably 8-week intervals). Vaccination usually begins at 2 months, but may be started as early as 6 weeks of age. Preterm infants should be vaccinated according to their chronological age from birth.
- Note: Pediarix is approved for the first 3 doses of polio vaccine. Per the ACIP, polio vaccine is given at 2, 4, and 6 to 18 months of age. Use of the minimum age and minimum intervals during the first 6 months of life should only be done when the vaccine recipient is at risk for imminent exposure to circulating poliovirus (shorter intervals and earlier start dates may lead to lower seroconversion) (CDC 58[30] 2009).
Previous vaccination with one or more components and scheduled to receive all vaccine components: Infants and Children <7 years:
- Hepatitis B vaccine: If previously vaccinated with 1 or 2 doses of another hepatitis B vaccine may use Pediarix to complete the 3-dose series. Not for use as birth dose of hepatitis B vaccine; infants who received a birth dose of hepatitis B vaccine may receive a 3-dose series of Pediarix (total of 4 hepatitis B vaccine doses). Infants born to HBsAg-positive women should begin dosing with DTaP-HepB-IPV by age 6 to 8 weeks after receiving the single antigen hepatitis B vaccine at birth (CDC/ACIP [Schillie 2018]).
- Diphtheria, tetanus toxoids, and acellular pertussis vaccine (DTaP): If previously vaccinated with 1 or 2 doses of Infanrix may use Pediarix to complete the first 3 doses of the series; use of Pediarix to complete DTaP vaccination started with products other than Infanrix has not been studied.
- Inactivated polio vaccine (IPV): If previously vaccinated with 1 or 2 doses of IPV may use Pediarix to complete the first 3 doses of the series.
仿單:
- 基礎接種:由二個月大開始,共接種3劑,每劑至少間隔1個月。
- 追加接種:基礎接種之後1年追加注射1劑,意即,通常在出生後18個月。
【Renal Impairment】
[Pediatric]
There are no dosage adjustments provided in the manufacturer`s labeling.
【Hepatic Impairment】
[Pediatric]
There are no dosage adjustments provided in the manufacturer`s labeling.
|
懷孕分級
Pregnancy Risk Factor |
Pediarix is not approved for use in patients >5 years of age.
-----------------------------------------------------------
[FDA(美國食品及藥物管理局)懷孕分級說明:
A:對照試驗無法證實懷孕初期及後期使用會危害胎兒。
B:動物試驗無法證實對胎兒有害,但缺乏人類對照試驗;或動物試驗有副作用報告,但無法證實對懷孕初期及後期之胎兒有害。
C:動物實驗中對胎兒有害但缺乏孕婦對照實驗;或無動物及孕婦試驗。
D:證實對胎兒有害,但疾病對孕婦有生命威脅或較安全藥品無法使用或無效時可使用。
X:證實對胎兒有害,且使用後危害大於可能的益處。孕婦及可能懷孕婦女禁用。]
|
| 禁忌症 |
1.先前接種白喉,破傷風,百日咳,b型嗜血桿菌,小兒麻痺相關疫苗或對本疫苗任何成分曾發生嚴重過敏反應者
2.漸進性腦病變者
3.先前接種含有百日核抗原(全細胞性或非細胞性百日咳疫苗)的疫苗後7天內曾發生腦病變,且無其他可解釋病因者
4.出生未滿六周
|
| Contraindications |
Hypersensitivity to diphtheria and tetanus toxoids, pertussis, hepatitis B, poliovirus vaccine, or any component of the vaccine; encephalopathy occurring within 7 days of a previous pertussis vaccine (not attributable to another identifiable cause); progressive neurologic disorders (including infantile spasms, uncontrolled epilepsy, or progressive encephalopathy).
|
| 常見副作用 | |
| Common adverse drug reactions | |
| Adverse Reactions |
The following adverse drug reactions and incidences are derived from product labeling unless otherwise specified.
Adverse events reported within 4 days of vaccination at 2-, 4-, and 6 months of age in patients given Pediarix concomitantly with Hib conjugate vaccine and PCV7 vaccine.
>10%:
Central nervous system: Irritability (?61% to 65%; grade 3: ?3% to 4%), drowsiness (41% to 57%)
Gastrointestinal: Anorexia (26% to 31%; grade 3: <1%)
Local: Erythema at injection site (25% to 40%; >20 mm: 1% to 3%), pain at injection site (31% to 36%; grade 3: 2% to 3%), swelling at injection site (17% to 29%; >20 mm: 2% to 3%)
Miscellaneous: Fussiness (<=61% to 65%; grade 3: 3% to 4%), fever (<=100.4°F: 28% to 39%; >=103.1°F: <=1%)
<1%, postmarketing, and/or case reports: Abnormal hepatic function tests, anaphylactoid reaction, anaphylaxis, angioedema, apnea, arthus phenomenon, bulging fontanel, cough, cranial nerve dysfunction (cranial mononeuropathy), crying, cyanosis, demyelinating disease, diarrhea, dyspnea, encephalitis, erythema, fatigue, febrile seizures, Guillain-Barre syndrome, hypersensitivity reaction, hypotonia, hypotonic/hyporesponsive episode, impaired consciousness, injection site reaction (cellulitis at injection site, induration at injection site, injection site nodule, injection site pruritus, injection site vesicle, warm sensation at injection site), insomnia, lethargy, limb pain, nervousness, neuritis (brachial), pallor, peripheral neuropathy (mononeuropathy), petechiae, restlessness, screaming, seizure, skin rash, sudden infant death syndrome, swelling of extremities, upper respiratory tract infection, urticaria, vomiting
|
★高警訊藥品
監測建議 |
|
監測
Monitoring |
Monitor for syncope for 15 minutes following administration (ACIP [Ezeanolue 2020]). If seizure-like activity associated with syncope occurs, maintain patient in supine or Trendelenburg position to reestablish adequate cerebral perfusion.
|
| 警語與注意事項 | |
| Warnings & precautions | |
| 針劑溶解條件 |
|
| 針劑稀釋條件 |
|
| 針劑不相容性 |
因為缺乏相容性的研究,因此本品不得與其他藥物混合使用。(1090918 仿單資料)
|
| 針劑施打條件 |
以肌肉注射方式接種。最好注射在大腿的前外側(中間三分之一的範圍)。(1080129仿單資料)
|
| 針劑保存安定性 |
儲存於2-8℃,不可冷凍。(1080129仿單資料)
|
最近修改日期時間
Updated | 5/28/2021 3:11:59 PM |
|
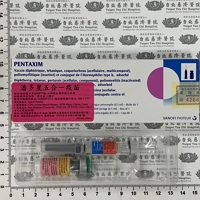
現用藥品
Available
|
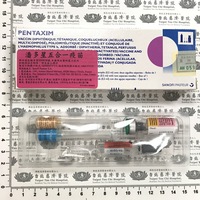
停用藥品
Old item
|

藥品仿單
DrugLabeling
|

二維條碼
QR code
|
|