藥品名稱
drug name | VARIVAX 0.5 mL/vial (Varicella Virus Vaccine), 伏痘敏活性水痘疫苗 (自費) |
| 藥檔狀態 | 使用中 |
成 份
Ingredient | Varicella Virus Vaccine |
| 單位含量 | 0.5 mL/vial |
| Dosage Forms | Injection, powder for reconstitution [preservative free]: 0.5 mL |
| 外觀描述 | |
| Appearance | |
廠商名稱
Manufacturer | 美商默沙東藥廠股份有限公司台灣分公司 |
製 造 商
Manufacturer | MERCK SHARP & DOHME CORP. |
字 號
Product ID | 衛署菌疫輸字第000480號 |
藥理分類
Pharmacologic Category | Vaccine;Vaccine, Live (Viral) |
作用機轉
Mechanism of action | As a live, attenuated vaccine, varicella virus vaccine offers active immunity to disease caused by the varicella-zoster virus by inducing cell mediated and humoral immune responses |
| 用途/適應症 | |
| Use |
Varicella prevention: For the prevention of varicella in persons 12 months and older
The Advisory Committee on Immunization Practices (ACIP) recommends vaccination for all children, adolescents, and adults who do not have evidence of immunity (CDC/ACIP [Marin, 2007]). Vaccination is especially important for:
‧ Health care personnel
‧ Household contacts of immunocompromised persons
‧ Persons living or working in environments where transmission is likely (teachers, child-care workers, residents and staff of institutional settings)
‧ Persons in environments where transmission has been reported
‧ Nonpregnant women of childbearing age
‧ Adolescents and adults in households with children
‧ International travelers
Varilrix (Canadian product): Also approved for use in high-risk patients (eg, acute leukemia [complete remission and lymphocyte count≧1,200/mm3 or that no other evidence of lack of cellular immune competence exists], planned organ transplantation [6 to 8 weeks prior to], receiving immunosuppressive treatment [with lymphocyte count ≧1,200/mm3], or other chronic diseases); approved for use in susceptible healthy contacts of high-risk patients.
Use: Off-Label: Adult
Varicella postexposure prophylaxis
|
衛福部核准適用症狀
MOHW approved indications |
水痘之主動免疫。
|
| 常用劑量 |
(藥品劑量會因人或病情增減,請依照醫師指示服用。) |
| Dose |
【Adult】
Varicella immunization: SubQ:
- US labeling: Two doses of 0.5 mL separated by≧4 weeks (4 to 8 weeks apart per ACIP). Note: The ACIP recommends that all children and adults without evidence of immunity receive 2 doses of the vaccine; those who received only 1 dose of a varicella-containing vaccine receive a second dose (CDC/ACIP [Marin 2007]).
- Canadian labeling: Two single doses 0.5 mL separated by 4 to 8 weeks (Varivax III) or≧6 weeks (Varilrix); Note: The NACI recommends that adolescents (≧13 years of age) and adults (<50 years of age) who received only 1 dose of vaccine receive a second dose (NACI 2016).
Varicella postexposure prophylaxis (healthy, previously unvaccinated individuals) (off-label use): SubQ: 0.5 mL administered ideally within 72 hours postexposure but may be used up to 120 hours (5 days) postexposure (CDC/ACIP [Marin 2007])
【Pediatric】
Primary immunization:
- CDC/ACIP recommendations: Children≧12 months: SubQ: 0.5 mL per dose for a total of 2 doses administered as follows: 12 to 15 months of age and 4 to 6 years of age. The second dose may be administered earlier provided≧3 months have elapsed after the first dose. If the second dose was administered≧4 weeks after the first dose, it may be considered as valid (CDC/ACIP [Marin 2007]).
- Canadian labeling: National Advisory Committee on Immunization (NACI) recommends 2 doses of 0.5 mL with first dose administered at 12 to 15 months of age. Separate doses by≧3 months; however, if rapid protection is necessary, may administer second dose after ≧4 weeks (NACI 2016).
>> Children:
* Varilrix: SubQ: 2 doses of 0.5 mL separated by≧6 weeks.
* Varivax III: SubQ: 0.5 mL as a single dose.
>> Adolescents: SubQ: 2 doses of 0.5 mL separated by 4 to 8 weeks (Varivax III) or≧6 weeks (Varilrix); Note: The NACI recommends that adolescents who received only 1 dose of vaccine receive a second dose (NACI 2016).
Catch-up immunization: Children and Adolescents: ACIP recommendations (CDC/ACIP [Marin 2007]): Note: Do not restart the series. If doses have been given, begin the below schedule at the applicable dose number. SubQ: 0.5 mL per dose for a total of 2 doses administered as follows:
- First dose given on the elected date.
- Second dose given at least 3 months after the first dose (if age <13 years) or at least 4 weeks after the first dose (if age≧13 years).
Varicella postexposure prophylaxis (healthy, previously unvaccinated individuals): Children (≧12 months) and Adolescents: SubQ: 0.5 mL administered ideally within 72 hours postexposure but may be used up to 120 hours (5 days) postexposure (CDC/ACIP [Marin 2007]).
【Geriatric】
Refer to adult dosing.
【Renal Impairment】
There are no dosage adjustments provided in the manufacturer`s labeling
【Hepatic Impairment】
There are no dosage adjustments provided in the manufacturer`s labeling
|
懷孕分級
Pregnancy Risk Factor |
依文獻內容判定系統稽核懷孕分級建置為:X
UpToDate:
Varicella virus vaccine is contraindicated for use in pregnant patients.
Information related to pregnancy and fetal outcomes following inadvertent exposure to the varicella virus vaccine was collected from 1995-2013 using the manufacturer`s pregnancy registry. Data was available from 905 women who received a varicella containing vaccine (30% within 3 months prior to conception) and who had known pregnancy outcomes. Among these women, the rates of miscarriage and birth defects was not increased above background rates, and there were no infants born with abnormalities consistent with congenital varicella syndrome. Postmarketing data collected since closure of the pregnancy registry has not detected new safety concerns or an increased risk of major birth defects following inadvertent varicella vaccine exposure during pregnancy (Woodward 2019).
Varicella disease during the first or second trimesters may result in congenital varicella syndrome. The onset of maternal varicella infection from 5 days prior to 2 days after delivery may cause varicella infection in the newborn. All women should be assessed for immunity during a prenatal visit; those without evidence of immunity should be vaccinated upon completion or termination of pregnancy (CDC/ACIP [Marin 2007]).
仿單:
目前尚未使用Varivax於動物的生殖研究,故目前未知懷孕婦女注射Varivax是否會對胎兒造成傷害,或影響其生殖能力,因此懷孕婦女不應注射Varivax。甚至注射疫苗後三個月內應避免懷孕。
-----------------------------------------------------------
[FDA(美國食品及藥物管理局)懷孕分級說明:
A:對照試驗無法證實懷孕初期及後期使用會危害胎兒。
B:動物試驗無法證實對胎兒有害,但缺乏人類對照試驗;或動物試驗有副作用報告,但無法證實對懷孕初期及後期之胎兒有害。
C:動物實驗中對胎兒有害但缺乏孕婦對照實驗;或無動物及孕婦試驗。
D:證實對胎兒有害,但疾病對孕婦有生命威脅或較安全藥品無法使用或無效時可使用。
X:證實對胎兒有害,且使用後危害大於可能的益處。孕婦及可能懷孕婦女禁用。]
|
| 禁忌症 |
1.對本疫苗所含之任何成分、包括gelatin發生過敏的人。
2.對neomycin有過敏病史的病人。
3.有罹患惡血值、血癌、任何一種淋巴癌,或其他影響骨髓或淋巴系統惡性腫瘤的病人。
4.正在接受免疫抑制劑治療的病人(包括使用皮質類固醇免疫抑制劑的病人)。
5.有先天或後天免疫功能不全的人,包含AIDS或感染其他具臨床意義的人類免疫缺乏病毒,細胞性免疫缺乏,免疫球蛋白過低或缺乏的免疫抑制病人。
6.先天或遺傳性免疫不全家族史的人,除非經證實具有足夠的免疫能力。
7.未經治療之活動性肺結核患者。
8.任何發燒性的呼吸系統疾病或其他活動性發燒感染。
9.懷孕。且接種疫苗後的三個月內要避免懷孕。
|
| Contraindications |
Severe allergic or anaphylactic reaction to the vaccine, a previous dose of a varicella-containing vaccine, or any component of the formulation, including neomycin and gelatin; immunosuppressed or immunodeficient individuals due to disease or medical therapy; active, untreated tuberculosis; active febrile illness with fever >38.5°C (>101.3°F); pregnancy or planning to become pregnant within the next 3 months.
Canadian labeling: Additional contraindications (not in US labeling): Varivax III: Family history of congenital or hereditary immunodeficiency (unless immune competence of vaccine recipient is demonstrated); Varilrix: Primary or acquired immunodeficiency with a total lymphocyte count <1,200/mm3.
|
| 常見副作用 | |
| Common adverse drug reactions | |
| Adverse Reactions |
>10%:
Local: Injection site reaction (19% to 33%; includes erythema, hematoma, induration, numbness, pain, swelling, stiffness)
Miscellaneous: Fever (10% to 15%)
1% to 10%:
Central nervous system: Chills, fatigue, headache, irritability, malaise, nervousness, sleep disturbance
Dermatologic: Varicella-like rash (≦6%; at injection site and generalized), contact dermatitis, dermatitis (children), diaper rash (children), eczema (children), miliaria (children), pruritus, skin rash, urticaria, xeroderma (children)
Gastrointestinal: Abdominal pain, anorexia, aphthous stomatitis (adolescents & adults), constipation, dental discomfort (teething; children), diarrhea, nausea, vomiting
Genitourinary: Herpes labialis (adolescents & adults)
Hematologic & oncologic: Lymphadenopathy
Hypersensitivity: Hypersensitivity reaction
Neuromuscular & skeletal: Arthralgia, myalgia, neck stiffness
Ophthalmic: Eye discomfort
Otic: Otitis
Respiratory: Cough, respiratory tract disease (lower/upper)
<1%, postmarketing, and/or case reports: Anaphylactic shock, anaphylaxis, angioedema, anterior uveitis (children; Krall 2014), aplastic anemia, aseptic meningitis, ataxia, Bell palsy, cellulitis, cerebrovascular accident, dizziness, encephalitis, erythema multiforme, facial edema, febrile seizures, Guillain-Barre syndrome, Henoch-Schonlein purpura, herpes zoster, immune thrombocytopenia, impetigo, keratitis (children; Krall 2014), meningitis, necrotizing retinitis (immunocompromised patients), paresthesia, peripheral edema, pharyngitis, pneumonia, pneumonitis, secondary skin infection, seizure (nonfebrile), Stevens-Johnson syndrome, thrombocytopenia, transverse myelitis, varicella infection - chickenpox (including virus transmission to susceptible contacts)
|
★高警訊藥品
監測建議 |
|
監測
Monitoring |
Rash, fever; monitor for anaphylaxis and syncope for 15 minutes following administration (ACIP [Ezeanolue 2020]). If seizure-like activity associated with syncope occurs, maintain patient in supine or Trendelenburg position to reestablish adequate cerebral perfusion.
|
| 警語與注意事項 | |
| Warnings & precautions | |
| 針劑溶解條件 |
以所附稀釋液(0.7mL)注入溶解。(1100422仿單資料)
|
| 針劑稀釋條件 |
|
| 針劑不相容性 |
|
| 針劑施打條件 |
需皮下注射,不可直接注射於血管內。最適合注射的部位在上臂三角肌外側、或大腿前外側。(1100422仿單資料)
|
| 針劑保存安定性 |
疫苗在與稀釋液混合前需冷藏存放在2-8℃或更低溫下(一旦移至冷藏,即不可再次冷凍);在與稀釋液混合之前,需避光儲存。
疫苗與稀釋液混合後需於30分鐘內使用完,否則應予以丟棄。(1100422仿單資料)
|
最近修改日期時間
Updated | 5/11/2021 4:09:11 PM |
|
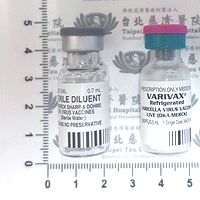
現用藥品
Available
|
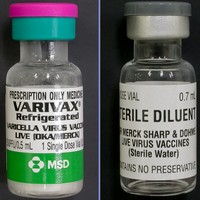
停用藥品
Old item
|

藥品仿單
DrugLabeling
|

二維條碼
QR code
|
|