藥品名稱
drug name | Influenza Virus Vaccine 0.5 ml/syringe (AdimFlu-S) 107年度公費流感疫苗 |
| 藥檔狀態 | |
成 份
Ingredient | Influenza virus vaccine |
| 單位含量 | 0.5 ml/syringe |
| Dosage Forms | Suspension Prefilled Syringe, Intramuscular : 0.5mL/syringe |
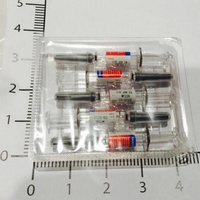
| 外觀描述 | 針劑/針筒 |
| Appearance | injection/syringe |
廠商名稱
Manufacturer | 賽諾菲股份有限公司 |
製 造 商
Manufacturer | SANOFI PASTEUR |
字 號
Product ID | 衛署菌疫製字第000113號 |
藥理分類
Pharmacologic Category | Vaccine; Vaccine, Inactivated (Viral) |
作用機轉
Mechanism of action | Promotes immunity to seasonal influenza virus by inducing specific antibody production. Each year the formulation is standardized according to the U.S. Public Health Service. Preparations from previous seasons must not be used. |
| 用途/適應症 | 流行性感冒疫苗 |
| Use |
Influenza disease prevention:Active immunization against influenza disease caused by influenza virus subtypes A and type B contained in the vaccine
|
衛福部核准適用症狀
MOHW approved indications |
預防流感。
|
| 常用劑量 | 立刻使用0.5mL
(藥品劑量會因人或病情增減,請依照醫師指示服用。) |
| Dose |
Adult
It is important to note that influenza seasons vary in their timing and duration from year to year. In general, vaccination should begin soon after the vaccine becomes available (and, if possible, by the end of October in the US) and prior to onset of influenza activity in the community. However, vaccination should continue throughout the influenza season as long as vaccine is available. The CDC does not recommend revaccination later in the season for those persons who have already been fully vaccinated. Unless noted, the ACIP does not have a preference for any given inactivated influenza vaccine (IIV) formulation when used within their specified age indications (CDC/ACIP [Grohskopf 2018)]. International considerations: Products with similar names but containing different strains may be circulating globally due to differences in recommendations between northern and southern hemisphere countries. In addition, recommendations related to use of influenza vaccines and approved ages may vary per country.
仿單︰
通常採皮下或肌肉注射。
3 歲以上兒童及成人:一劑0.5 ml
Pediatric
It is important to note that influenza seasons vary in their timing and duration from year to year. In general, vaccination should begin soon after the vaccine becomes available such that the patient is fully immunized prior to onset of influenza activity in the community (eg, by end of October in US). However, vaccination should continue throughout the influenza season as long as vaccine is available. Unless noted, the ACIP and AAP do not have a preference for any given inactivated influenza vaccine (IIV) formulation when used within their specified age indications (AAP 2018; CDC/ACIP [Grohskopf 2018)].International considerations: Products with similar names but containing different strains may be circulating globally due to differences in recommendations between northern and southern hemisphere countries. In addition, recommendations related to use of influenza vaccines and approved ages may vary per country.
Note: Infants and children 6 months to <9 years of age who received at least 2 doses of trivalent or quadrivalent influenza vaccine prior to July 1, 2018 need only 1 dose of the 2018 to 2019 seasonal influenza vaccine. The 2 doses need not have been received during the same season or consecutive seasons. All other children <9 years of age (including those whose vaccination status cannot be determined) should receive 2 doses separated by 4 weeks in order to achieve satisfactory antibody response (CDC/ACIP [Grohskopf 2018]).
仿單:
通常採皮下或肌肉注射。
0.5歲以上未滿3歲兒童︰一劑0.25mL
3 歲以上兒童及成人:一劑0.5 ml
未滿 9 歲兒童,若先前未曾注射過流感疫苗者,需接種 2 次,且間隔至少 4 週。
Geriatric
It is important to note that influenza seasons vary in their timing and duration from year to year. In general, vaccination should begin soon after the vaccine becomes available (and, if possible, by October) and prior to onset of influenza activity in the community. However, vaccination should continue throughout the influenza season as long as vaccine is available. The high-dose inactivated influenza vaccine (IIV), Fluzone High-Dose, contains 60 mcg of each vaccine antigen per 0.5 mL dose compared to 15 mcg of each vaccine antigen per 0.5 mL dose. The high-dose IIV formulation was shown to elicit a higher antibody response and may provide better protection against influenza illness compared to standard dose IIV3 formulations. However, the ACIP does not have a preference for any given IIV formulation in older adults when used within their specified age indications (CDC/ACIP [Grohskopf 2018]).
仿單:
3 歲以上兒童及成人:一劑0.5 ml
Renal impairment
There are no dosage adjustments provided in the manufacturer’s labeling.
Hepatic impairment
There are no dosage adjustments provided in the manufacturer’s labeling.
|
懷孕分級
Pregnancy Risk Factor |
FDA: B/C (manufacturer specific) 。
仿單:已有超過2000位孕婦接種季節性流感疫苗的研究報告顯示並無任何胎兒產生不良影響。但本疫苗並無針對動物生殖系統的研究亦無接種於孕婦是否傷害胎兒或影響生殖能力的報告。由於孕婦是流感併發症的高危險族群且可在接種疫苗後提供胎兒保護力,除孕婦已知(或曾有)對本劑成分或雞蛋、雞肉、亦或其他雞來源物有過敏者外,建議孕婦應依流感流行的適用性優先施打本疫苗。
-----------------------------------------------------------
[FDA(美國食品及藥物管理局)懷孕分級說明:
A:對照試驗無法證實懷孕初期及後期使用會危害胎兒。
B:動物試驗無法證實對胎兒有害,但缺乏人類對照試驗;或動物試驗有副作用報告,但無法證實對懷孕初期及後期之胎兒有害。
C:動物實驗中對胎兒有害但缺乏孕婦對照實驗;或無動物及孕婦試驗。
D:證實對胎兒有害,但疾病對孕婦有生命威脅或較安全藥品無法使用或無效時可使用。
X:證實對胎兒有害,且使用後危害大於可能的益處。孕婦及可能懷孕婦女禁用。]
|
| 禁忌症 |
被接種者有下列任何一狀態時,不可接種:
(1)先前接種本疫苗或對本疫苗任何成分曾發生嚴重過敏反應者。
(2)發燒或正患有急性中重度疾病者,宜待病情穩定後再接種。
(3)出生未滿6個月不適合接種。
(4)先前接種本疫苗六週內曾發生格林-巴利症候群(Guillain-Barre syndrome, GBS)者。
(5)已知對「蛋」之蛋白質有嚴重過敏者,可在門/住診由熟悉處理過敏症狀之醫事人員提供接種,並於接種後觀察30分鐘,無不適症狀再離開。
(6)其他經醫師評估不適合接種者。
|
| Contraindications |
Severe allergic reaction (eg, anaphylaxis) to a previous influenza vaccination; hypersensitivity to any component of the formulation.
|
| 常見副作用 | 可能發生:注射部位出現疼痛/痠痛、腫脹、發紅、手臂活動困難,鼻塞、咳嗽、喉嚨痛、胸悶、肌肉痛、全身無力、頭痛、疲倦(駕車或機器操作注意)、發燒。 |
| Common adverse drug reactions | Injection site reactions(pain, soreness, swelling, erythma, myalgia), nasal congestion, cough, oropharyngeal pain, chest tightness, muscle pain, arthralgia, headache, fatigue(Be careful driving or operating machines) and fever. |
| Adverse Reactions |
Frequency not defined. Adverse reactions in adults ≧65 years of age may be greater using the high-dose vaccine, but are typically mild and transient.
Cardiovascular: Chest tightness, hypertension
Central nervous system: Chills, drowsiness, fatigue, headache, irritability, malaise, migraine, shivering
Dermatologic: Diaphoresis, ecchymoses
Gastrointestinal: Abdominal pain, decreased appetite, diarrhea, gastroenteritis, nausea, sore throat, vomiting
Infection: Infection, varicella
Local: Injection site reactions (including bruising, erythema, hematoma at injection site, induration, inflammation, itching at injection site, pain, rash, soreness, swelling at injection site, tenderness at injection site)
Neuromuscular & skeletal: Arthralgia, back pain, myalgia (may start within 6 to 12 hours and last 1 to 2 days; incidence generally equal to placebo in adults; occurs more frequently than placebo in children)
Respiratory: Bronchitis, cough, dyspnea, nasal congestion, nasopharyngitis, oropharyngeal pain, pharyngitis, pharyngolaryngeal pain, rhinitis, rhinorrhea, upper respiratory tract infection, wheezing
Miscellaneous: Fever
|
★高警訊藥品
監測建議 |
|
監測
Monitoring |
Monitor for anaphylaxis and syncope for 15 minutes following administration (ACIP [Kroger 2017]). If seizure-like activity associated with syncope occurs, maintain patient in supine or Trendelenburg position to reestablish adequate cerebral perfusion.
|
| 警語與注意事項 | 肌肉注射。可能會造成發燒或過敏反應。冷藏避光儲存。107.10.18公費流感疫苗為AdimFlu-S。 |
| Warnings & precautions | For injection. Store in the refrigerator. Protect from light. |
| 針劑溶解條件 |
|
| 針劑稀釋條件 |
|
| 針劑不相容性 |
不得將流感疫苗和其他藥物混合在同一個注射筒內。(1071017仿單資料)
|
| 針劑施打條件 |
通常採皮下或肌肉注射。應待疫苗回到室溫後再施打(1071017仿單資料)
|
| 針劑保存安定性 |
避光冷藏儲存(2°C ~ 8°C) ,不可冷凍。如果疫苗已結凍,不可使用。(1071017仿單資料)
|
最近修改日期時間
Updated | 12/21/2018 3:13:28 PM |
|

現用藥品
Available
|
停用藥品
Old item
|

藥品仿單
DrugLabeling
|
|

二維條碼
QR code
|
|