藥品名稱
drug name | Measles,Mumps,Rubella Virus Vaccine 0.5ml/vial (M-M-R II, 麻疹、腮腺炎、德國麻疹疫苗) 自費 |
| 藥檔狀態 | |
成 份
Ingredient | Measles, Mumps, Rubella Virus Vaccine |
| 單位含量 | 0.5ml/vial |
| Dosage Forms | 0.5ml/vial |
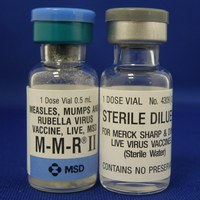
| 外觀描述 | 針劑/小瓶 |
| Appearance | injection/vial |
廠商名稱
Manufacturer | 美商默沙東藥廠股份有限公司台灣分公司 |
製 造 商
Manufacturer | MERCK & CO. Inc. |
字 號
Product ID | 衛署菌疫輸字第000364號 |
藥理分類
Pharmacologic Category | Biologic and Immunologic Agents/ Immunizations/ Vaccines-Virus (Live) |
作用機轉
Mechanism of action | As a live, attenuated vaccine, MMR vaccine offers active immunity to disease caused by the measles, mumps, and rubella viruses. |
| 用途/適應症 | 麻疹、腮腺炎、德國麻疹疫苗 |
| Use |
To provide simultaneous active immunization against measles, mumps, rubella, and varicella.
|
衛福部核准適用症狀
MOHW approved indications |
預防麻疹、腮腺炎、德國麻疹。
|
| 常用劑量 | 立刻使用0.5mL
(藥品劑量會因人或病情增減,請依照醫師指示服用。) |
| Dose |
Immunization: SubQ: 0.5 mL
Birth year in or after 1957 without evidence of immunity: 1 or 2 doses (0.5 mL/dose); minimum interval between doses is 28 days.
Adults born in or after 1957 without documentation of live vaccine on or after first birthday, or without physician-diagnosed measles or mumps, or without laboratory evidence of immunity, should be vaccinated with at least one dose; a second dose, separated by no less than 1 month, is indicated for those previously vaccinated with one dose of measles vaccine, students entering institutions of higher learning, recently exposed in an outbreak setting, healthcare workers at time of employment, and for travelers to endemic areas (CDC, 1998).
Persons vaccinated between 1963 and 1967 with a killed measles vaccine, followed by live vaccine within 3 months, or with a vaccine of unknown type should be revaccinated with live measles virus vaccine. Persons vaccinated before 1979 with either a killed mumps vaccine or a mumps vaccine of unknown type should be considered for revaccination with two doses of MMR (CDC, 1998).
Women of childbearing potential, without documentation of rubella immunity, regardless of birth year, should also receive one dose of vaccine. Do not administer rubella to women who are or who may become pregnant within 1 month of receiving vaccine; administer following completion or termination of pregnancy (CDC, 1998).
Healthcare personnel (unvaccinated) born prior to 1957 and who are without laboratory evidence of measles, mumps, and/or rubella immunity or laboratory confirmation of disease: Consider 2 doses of MMR vaccine at the appropriate interval. Two doses of the MMR vaccine are needed for measles and mumps, one dose is needed for rubella (CDC, 2006).
|
懷孕分級
Pregnancy Risk Factor |
XM,FDA:C
-----------------------------------------------------------
[FDA(美國食品及藥物管理局)懷孕分級說明:
A:對照試驗無法證實懷孕初期及後期使用會危害胎兒。
B:動物試驗無法證實對胎兒有害,但缺乏人類對照試驗;或動物試驗有副作用報告,但無法證實對懷孕初期及後期之胎兒有害。
C:動物實驗中對胎兒有害但缺乏孕婦對照實驗;或無動物及孕婦試驗。
D:證實對胎兒有害,但疾病對孕婦有生命威脅或較安全藥品無法使用或無效時可使用。
X:證實對胎兒有害,且使用後危害大於可能的益處。孕婦及可能懷孕婦女禁用。]
|
| 禁忌症 |
1.對此疫苗之任何成份包括動物膠有過敏者。2.孕婦勿接種M-M-R II;此疫苗對胎兒發展的可能影響在此階段尚未知曉。青春期後女性接種此疫苗之後三個月內必須避免懷孕。3.對neomycin會產生過敏或類過敏反應者(稀釋後每劑量疫苗內約25微克之neomycin)。4.任何發燒的呼吸道疾病或其他活動性發燒感染。5.活動性未治療的肺結核。6.接受免疫抑制療法患者不可接種,但使用皮質類固醇作為置換療法如愛迪生氏病(Addison,s disease)之患者,不在此限。7.惡血質病、白血病、任何類型的淋巴瘤,其他影響骨髓或淋巴系統的惡性腫瘤患者。8.原發性及後天性免疫缺乏情況,包括因AIDS或其他人類免疫缺乏病毒感染之免疫抑制患者細胞性免疫缺乏;及低丙種球蛋白血症(hypopammaglobulinemic)與血中兩種球蛋白功能不良症(dysgammaglobulinemic)。有報告指出,當含麻疹病毒的疫苗不慎被使用於嚴重的免疫力妥協病人時,此疫苗中散佈的麻疹病毒直接造成麻疹包含性腦炎(measles inclusion body encephalitis, MIBE),肺炎及死亡。9.有先天性或遺傳性免疫缺乏家族史的個人,在其免疫能力被確定前。
|
| Contraindications |
Hypersensitivity to measles, mumps, and/or rubella vaccine or any component of the formulation; current febrile respiratory illness or other febrile infection; patients receiving immunosuppressive therapy (does not include corticosteroids as replacement therapy); primary and acquired immunodeficiency states; individuals with blood dyscrasias, leukemia, lymphomas, or other malignant neoplasms affecting the bone marrow or lymphatic systems; family history of congenital or hereditary immunodeficiency (until immune competence in the vaccine recipient is demonstrated); pregnancy.
|
| 常見副作用 | 可能發生:注射部位紅腫、疼痛、發燒、發疹。 |
| Common adverse drug reactions | Injection site erythem, pain, fever and rash. |
| Adverse Reactions |
Frequency not defined:
Cardiovascular: Syncope, vasculitis
Central nervous system: Ataxia, dizziness, febrile seizure, fever, encephalitis, encephalopathy, Guillain-Barre syndrome, headache, irritability, malaise, measles inclusion body encephalitis, polyneuritis, polyneuropathy, seizure, subacute sclerosing panencephalitis,
Dermatologic: Angioneurotic edema, erythema multiforme, measles-like rash, pruritus, purpura, rash, Stevens-Johnson syndrome, urticaria
Endocrine & metabolic: Diabetes mellitus, parotitis
Gastrointestinal: Diarrhea, nausea, pancreatitis, sore throat, vomiting
Genitourinary: Epididymitis, orchitis
Hematologic: Leukocytosis, thrombocytopenia
Local: Injection site reactions which include burning, induration, redness, stinging, swelling, tenderness, wheal and flare, vesiculation
Neuromuscular & skeletal: Arthralgia/arthritis (variable; highest rates in women, 12% to 26% versus children, up to 3%), myalgia, paresthesia
Ocular: Conjunctivitis, ocular palsies, optic neuritis, papillitis, retinitis, retrobulbar neuritis
Otic: Nerve deafness, otitis media
Renal: Conjunctivitis, retinitis, optic neuritis, papillitis, retrobulbar neuritis
Respiratory: Bronchospasm, cough, pneumonia, pneumonitis, rhinitis
Miscellaneous: Anaphylactoid reactions, anaphylaxis, atypical measles, panniculitis, regional lymphadenopathy.
|
★高警訊藥品
監測建議 |
|
監測
Monitoring |
Monitor for syncope for 15 minutes following administration. If seizure-like activity associated with syncope occurs, maintain patient in supine or Trendelenburg position to reestablish adequate cerebral perfusion.
|
| 警語與注意事項 | 冷藏貯存避光。泡製後可放8hrs。孕婦禁用。接種後三個月內應避免懷孕。為確保品質,領藥後無法接受退藥。 |
| Warnings & precautions | 冷藏貯存避光。泡製後可放8hrs。孕婦禁用。接種後三個月內應避免懷孕。為確保品質,領藥後無法接受退藥。 |
| 針劑溶解條件 |
稀釋時,只使用與疫苗提供的稀釋液。因其不含防腐劑及其他會使疫苗失去活性的物質。(1020111仿單資訊)
|
| 針劑稀釋條件 |
|
| 針劑不相容性 |
|
| 針劑施打條件 |
供皮下注射。(1020111仿單資訊)
|
| 針劑保存安定性 |
稀釋前疫苗應貯存於溫度2-8℃以下。稀釋液可與疫苗一起貯存於冰箱中或將其單獨置於室溫中,切勿冷凍。
稀釋後請儘快使用。若需貯存,則將此稀釋後的疫苗置於其原來小瓶中貯存於2-8℃黑暗處。若於8小時內未使用完即需丟棄。(1020111仿單資訊)
|
最近修改日期時間
Updated | 5/7/2019 9:41:17 AM |
|

現用藥品
Available
|
停用藥品
Old item
|

藥品仿單
DrugLabeling
|
|

二維條碼
QR code
|
|